food additives & acronyms
a 5 minute summary of the "generally recognized as safe" (GRAS) loophole and why it matters
Have you been hearing a lot in the news lately about food additives and "generally recognized as safe" ingredients, aka GRAS? Given current players in our political landscape, that is not surprising. Here is the lowdown…
Why this matters
Ingredients classified as Generally Recognized as Safe (GRAS) are pervasive in our food supply. Let’s look at one common ingredient as an example. Potassium bromate, a dough strengthener, is deemed by the International Agency for Research on Cancer (IARC) to be “potentially carcinogenic for humans” yet is allowable in food in the United States as it is classified as GRAS. Potassium bromate is banned around the world including, but not limited to, the European Union, Canada, and India. When the consumer purchases packaged bread with ultra-processed ingredients, the initial sticker cost does not accurately reflect the true cost when you factor in the overall health impacts. To understand how we are in this situation, we need to look at the regulations that made this permissible.
The GRAS Loophole
The Federal Food, Drug, and Cosmetic Act (“the Act”) of 1958 states that “any substance that is intentionally added to food is a food additive, that is subject to premarket review and approval by FDA, unless the substance is generally recognized, among qualified experts, as having been adequately shown to be safe under the conditions of its intended use, or unless the use of the substance is otherwise excepted from the definition of a food additive.” Originally, this GRAS classification was intended to streamline the process for more innocuous ingredients such as spices and vinegars. Up until 1997, ingredients were classified as GRAS only if they went through a lengthy GRAS Affirmation Petition Process culminating with the FDA issuing a Final Rule regarding the status of submitted ingredients.
As the years progressed, the number of ingredients manufacturers submitted for review skyrocketed. To ease the administrative burden of this process, the FDA modified the GRAS procedure in 1997 by removing the mandatory notification and approval process. The notification procedure became voluntary and thus food manufacturers were granted the authority to designate an ingredient as GRAS, no FDA approval required. This means that both consumers and the FDA do not have a clear picture of everything in our food supply, let alone any adverse health outcomes as a result of consuming those ingredients. Unsurprisingly, food manufacturers take full advantage of this.
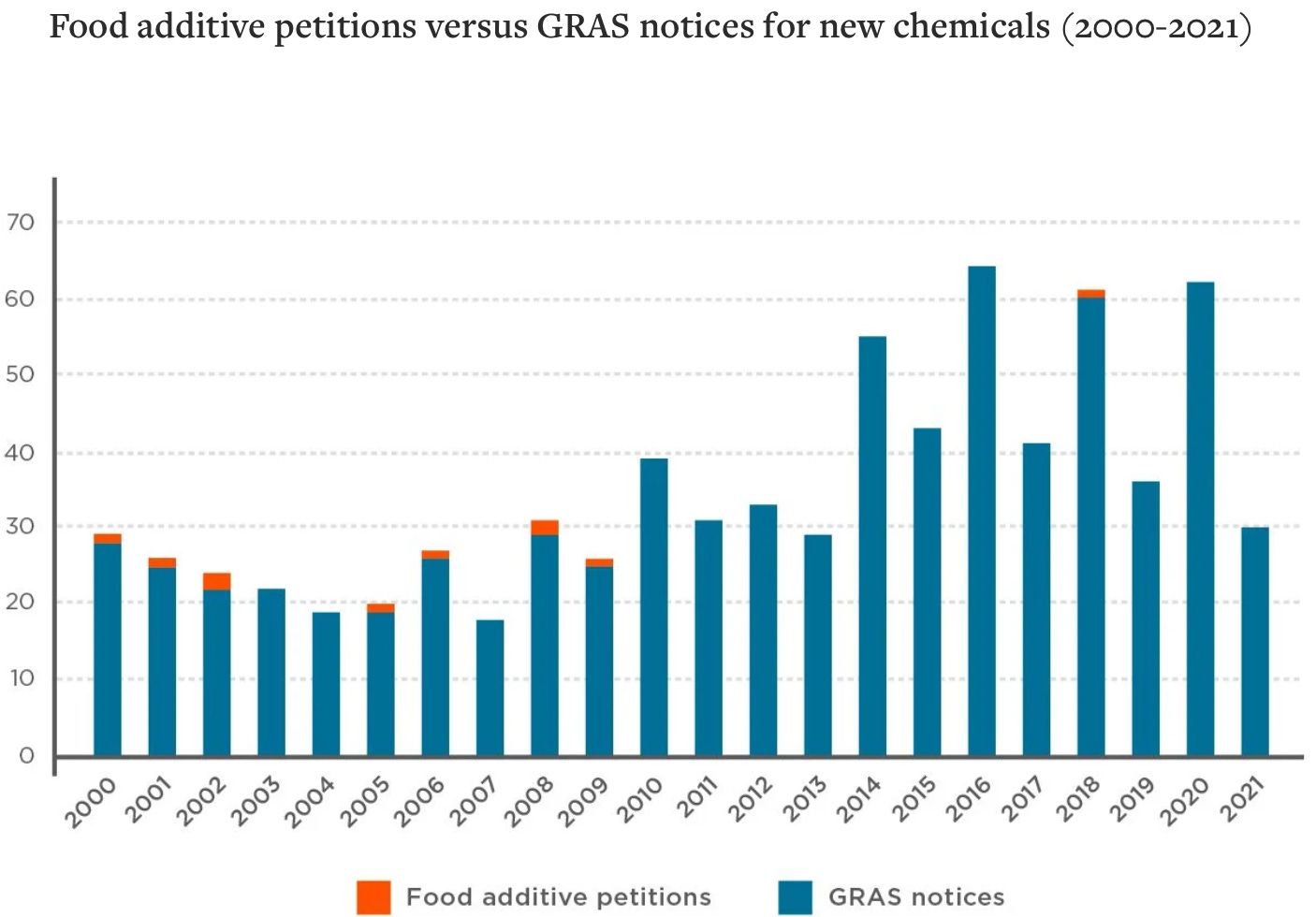
There is a critical distinction in the 1958 Act between “food additives” and GRAS ingredients. The former maintains a more rigorous FDA petition process, including pre-market safety review of the chemical. The issue is that food manufacturers are not filing new ingredients as food additives now that the GRAS loophole is accessible to them. Analysis conducted by the Environmental Working Group (EWG) found that between 2000 and 2001, 756 of the 766 new ingredients logged by the FDA were submitted via GRAS review, with only ten submitted under food additive petitions (see chart below). Of those added as GRAS, 99% were not reviewed by the FDA. Note that those numbers account for only what was voluntarily reported by manufacturers.
One of the issues with this regulation is that while adding something to the GRAS list does not require FDA review, removing something requires an incredibly high threshold of evidence that proves the ingredient is overwhelmingly detrimental to human health. It can be argued that the reactive versus proactive approach feels antithetical to the stated mission of the FDA which is to be “responsible for protecting the public health…by ensuring the safety of our nation's food supply.” There have been a few instances where an ingredient has been removed, one being in 2015 when the FDA ruled that artificial trans fats were unsafe for human consumption, giving manufacturers three years to remove them from the food supply.
The amount of research on ultra-processed ingredients has grown in recent years, yet discerning which specific ingredients and which combinations cause the biggest health risks has been harder to pinpoint. “A growing body of data shows instances of exposure to combinations of multiple additives, which may have potential ‘cocktail effects’ with greater implications for human health than exposure to a single additive,” states a 2024 epidemiological study on ultra-processed food exposure and adverse health outcomes. As such, the FDA and policy makers have not made any a wholesale moves to ban ultra-processed ingredients since untangling the effects of an individual ingredient is proving to be difficult.
Now I want to hear from you! Did you realize that items in our food supply are always not vetted by the FDA? Do you think more should be done? Drop a comment below to let me know what you think.
Sources
Shanmugavel, Venu, et al. “Potassium Bromate: Effects on Bread Components, Health, Environment and Method of Analysis: A Review.” Food Chemistry, vol. 311, May 2020, p. 125964. https://doi.org/10.1016/j.foodchem.2019.125964.
“Generally Recognized as Safe (GRAS).” U.S. Food & Drug Administration, 10 Oct. 2023, www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras.
Backhaus, Olivia, and Melanie Benesh. “EWG analysis: Almost all new food chemicals greenlighted by industry, not the FDA.” Environmental Working Group, 13 Apr. 2022, www.ewg.org/news-insights/news/2022/04/ewg-analysis-almost-all-new-food-chemicals-greenlighted-industry-not-fda.
“Artificial Trans Fats Banned in U.S. | Harvard T.H. Chan School of Public Health.” Harvard T.H. Chan School of Public Health, 22 Nov. 2024, hsph.harvard.edu/news/us-bans-artificial-trans-fats.
Lane, Melissa M., et al. “Ultra-processed Food Exposure and Adverse Health Outcomes: Umbrella Review of Epidemiological Meta-analyses.” BMJ, Feb. 2024, p. e077310. https://doi.org/10.1136/bmj-2023-077310.





This is very informative. I didn't know anything about GRAS. I live in Canada, so I was glad to see potassium bromate on the no-go list here, but we share much of our food supply chain with the US, so I have the same worries.